The Summer Internship Partnership of ASA and Novartis Oncology: A Multi-Win Endeavor
William (Bill) Mietlowski is a biometrical fellow who has coordinated the ASA-sponsored summer internship program for Novartis Oncology Biometrics and Data Management since October 2006.
Some multi-party relationships are zero-sum games (winner and loser). Occasionally, some relationships are win-win. Rarely do more than two parties in a relationship benefit. However, I think the American Statistical Association and Novartis Oncology summer internship partnership is a win-win-win-win-win relationship:
- The intern benefits from exposure to real-world applied problems in a pharmaceutical setting, possible professional presentations and/or publications stemming from internship problems, and enhanced employment opportunities post-graduation.
- The university benefits from external support for their students outside the academic year, recognition for the university in professional presentations and publications by the intern, and potential new areas of research that might follow from some of the internship projects.
- The mentor benefits from increased understanding of the new methodology explored by the internship project, awareness of new literature and/or software, and co-authorship of the intern’s presentations and publications.
- The company benefits from potential improvement in drug development from successful internship projects and a potential source of new talent familiar with the corporate culture and environment.
- The American Statistical Association benefits from the recognition the organization receives at the corporate level from the quality of the internship candidates that apply based on the ad published in the December issue of Amstat News or on the ASA website.
I have had the privilege of working with the ASA on a summer internship program since October of 2006 on behalf of Biostatistics and Data Management (BDM) at Novartis Oncology. This article summarizes my experience for the first five years of our partnership and the types of activities summer interns experience. Eleven former interns from 2007–2010 were interviewed and their thoughts about how their summer internship affected their career plans are summarized below.
Internship Applications from 2007–2011
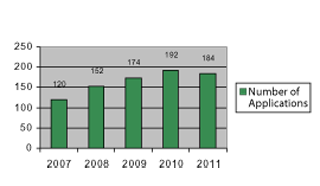
Novartis employed four statisticians as summer interns each year from 2007–2011 at its Florham Park, New Jersey, site and a fifth statistician as a summer intern at the Cambridge, Massachusetts, site from 2009–2011. Figure 1 depicts the number of internship applications received by internship year.
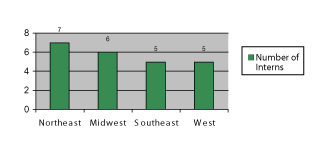
The internship employment rate from 2007–2011 was approximately 3% of applicants for each year. Figure 2 displays the distribution of the 23 selected interns by geographical region of the United States.
There appears to be good diversity of internship selection by geographical region of the United Status.
Intern Project Selection and Intern Selection
We solicit proposals for internship projects from all of our biostatisticians globally (both U.S. sites as well as Basel, Switzerland; Paris, France; Hyderabad, India; and Tokyo, Japan). Most importantly, the project must meet a business need. It may represent, but is not limited to, developing a new method based on real problems at Novartis Oncology, evaluating a new method proposed in the literature applied to actual or simulated clinical trial data, novel applications of existing methodology, or sensitivity analyses to determine the robustness of some of our standard methods to departures from assumptions. We select projects we would like to investigate personally, but cannot due to day-to-day project work.
Ideally, the projects should represent a learning experience for both the intern and mentor and be feasible for the intern to make substantial progress with during the 12-week internship period. After the project list is reviewed and finalized, the number of interns to be allocated at each site is determined. Based on the number of interns to be employed at the Florham Park and Cambridge sites and the nature of the projects to be assigned to each intern, a draft summer internship ad is prepared and the final ad is submitted to the ASA in October.
After the internship ad is published on the ASA website and in the internship issue of Amstat News, the internship applications are reviewed and potential candidates are identified based on goodness of fit to the proposed projects.
Internship Activities
Besides the projects, summer interns are welcome to participate in a variety of educational opportunities (e.g., seminars by internal and external speakers, lunch-and-learn presentations, etc.) within biostatistics and within the Oncology Business Unit (OBU) of Novartis. In 2010 and 2011, graduate interns within the OBU (e.g., master’s level, MBAs, Pharm D. candidates, PhD candidates) were assigned to cross-functional projects by the OBU Human Resources Department. This gave the interns an opportunity to understand the drug development process from another perspective and to educate their nonstatistical graduate internship colleagues about the role of a statistician in drug development.
This year, we plan to have a video conference linking the summer interns at the Basel, Florham Park, and Cambridge sites so they have an opportunity to meet each other and share information.
Finally, human resources organizes a variety of social activities for summer interns (e.g., tours of the manufacturing site, barbecues, bowling, etc.).
An Interview with 11 Former Summer Interns
To get an intern’s perspective of the impact of a summer internship on their career planning, we asked two questions of 11 former interns. Their open-ended responses are summarized below.
-
Question 1. What did the summer internship teach you about the kind of work you want to do after graduation?
- Clinical trial/development preference (4)
- Oncology preference (4)
- Industrial preference (3)
- Question 2. What nonstatistical training, experience, or insight did you obtain that shaped or benefited your future career development?
- Importance of communication with a variety of disciplines (7)
- Value of more biologic/subject matter knowledge (5)
- Awareness of complex multifunctional factors underlying drug development (2)
- Awareness of biomarker and genomic focus in oncology (1)
- Focus of employment interviews (40%–60%) on internship experience and projects (1)
- Handwritten letter from a patient that was moving and inspirational (1)
The post-graduate employment positions of the 11 interviewed summer interns are the following:
| Type of Employment | Number of Interns | Novartis Oncology BDM | 5 |
|---|---|
| Academia | 2 |
| Other pharmaceutical companies | 1 |
| Government | 1 |
| Novartis Modeling and Simulation | 1 |
| Still completing PhD requirements | 1 |
Internship Project–Based Presentations and Publications
Based on their internship projects, six interns have either made external presentations at professional meetings, had a published abstract for a professional meeting, had a manuscript published in a peer-reviewed journal, or have a manuscript in preparation.
-
Professional presentations: 6 (Joint Statistical Meetings, 5; Drug Information Association annual meeting, 1)
-
Abstract accepted for publication but not presentation: 1 (American Society for Clinical Oncology annual meeting)
-
Publications in peer-reviewed journals: 2 (Journal of Biopharmaceutical Statistics, Contemporary Clinical Trials)
- Manuscript in preparation: 1
Concluding Remarks
As more knowledge is gained about the nature of cancer, the disease appears to be even more complex and heterogeneous than thought. The tasks facing the statistician in oncology research may become more daunting and challenging. However, the talents of our emerging statisticians as embodied in the interns we have employed provide hope that these challenges will be met.