Meet Sylva Collins, Director of the Office of Biostatistics, US Food and Drug Administration
Sylva Collins joined the US Food and Drug Administration’s Office of Biostatistics in the Office of Translational Sciences of the Center for Drug Evaluation and Research on August 19, 2019. She brings more than 30 years of drug development experience and biostatistics leadership to her position.
Prior to joining the FDA, Collins was vice president of biometrics at ACADIA Pharmaceuticals, based in San Diego, where she led all aspects of statistical design and analysis of clinical trials, as well as statistical programming and clinical data management. She has emphasized standardization and automation to accelerate clinical development. She pioneered the large-scale deployment of electronic data capture at multiple companies, including Bayer and Novartis. She implemented standardization of biometrics systems and processes for large pharma organizations to allow near simultaneous regulatory submissions globally. She has experience leading large global biometrics organizations and has contributed to dozens of successful regulatory submissions in multiple jurisdictions.
Collins earned her bachelor’s degree in mathematics from the American University of Beirut, master’s and doctoral degrees in statistics from Boston University, and later a master’s degree in computer science from New York University.
Please tell us about your work as director of the Office of Biostatistics at the US Food and Drug Administration.
The Office of Biostatistics (OB) at FDA enjoys a reputation for excellence. Seeing it up close is even more impressive.
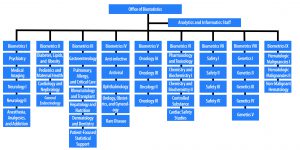
OB’s core mission is to ensure the safety and efficacy of drugs and biologics. Our staff reviews regulatory submissions for new drugs, therapeutic biologics, and generic products (NDAs, BLAs, and ANDAs). We check for data quality and completeness; perform thoughtful, state-of-the-art statistical analyses to determine the validity of the sponsor’s analyses; assess risk vs. benefit in support of product approval; and present findings to advisory committees. Once drugs are approved, our mission continues. We develop methods for post-marketing surveillance and review surveillance studies.
OB’s role in reviewing hundreds of applications is well known. However, our role extends beyond application review. Our statisticians advise sponsors on statistical approaches in study protocols. In 2019, OB reviewed more than 350 NDAs, BLAs, and ANDAs and provided advice on more than 2,500 study protocols.
There are nearly 250 analysts, statisticians, and support staff in OB. Almost all the staff hold advanced degrees in statistics or biostatistics. The majority have PhDs.
Our staff stays current on all relevant statistical developments. They conduct independent research on statistical methodologies relevant to Center for Drug Evaluation and Research’s (CDER’s) scientific mission and regulatory review process. Research includes such topics as Bayesian methods, meta-analysis, risk-benefit analysis, signal detection, and bioequivalence. In 2019, OB staff published more than 70 papers in peer-reviewed journals about these topics.
What about this position appealed to you, and what are your priorities for the next few years?
OB’s overarching objective is to maintain global scientific leadership and rigorous standards for statistical reviews of drug applications while striving constantly to be faster and more efficient.
Biostatistics is an open field. Our staff carries out independent research on statistical methods relevant to CDER’s mission. There are many ongoing and emerging topics in statistical design and analysis. There is strong motivation to reach more definitive conclusions in less time.
We are at the forefront of promoting complex innovative clinical trial designs and patient-focused drug development. An emerging topic is the use of real-world evidence (RWE) for regulatory decisions. Our statisticians organize public workshops, develop guidance documents for industry, and present about these topics at professional conferences.
We are speaking with industry, academia, and the patient community about innovative approaches to drug development and approval, including nontraditional data sources such as RWE, external controls, and Bayesian methods. These present interesting statistical challenges to exploit available data while avoiding bias and other statistical pitfalls.
It has been an exciting first few months. Coming up to speed on all the initiatives in OB has been a large but enjoyable experience. Just getting to know the staff members and their capabilities has been rewarding.
What do you like most about working as a statistician for the FDA? What’s most challenging?
There are two basic parts to the statistician’s job. One is to identify the relevant statistical methods needed to reach a useful conclusion. The other is getting it done.
OB is a focal point for statistical development. It is where statistical theory meets statistical practice. The development of new pharmaceuticals adds some dimensions to the question of statistical inference. In the practical world, we must always be cognizant of time and efficiency. Our overriding objective is to serve patients. We cannot ignore the cost in time and resources when working to improve strategies for drug development.
In these regards, this is an exciting time to be a statistician. In my position, I can contribute to the process of accelerating these new strategies.
The esteem with which OB is held in the industry is well known. Nonetheless, FDA does not have all the answers, nor does it have all the questions. At FDA, I can fully collaborate with industry and academia. OB not only reviews protocols and submissions, but also makes original contributions. It is stimulating to be at the intersection of statistical theory, clinical trial practice, and regulatory oversight.
What was your training and career path to your current position?
Prior to FDA, all my positions were in the pharmaceutical industry. These positions ranged across sponsors, contract research organizations, and software developers. My very first position at Lederle Laboratories came immediately after completing my PhD in mathematical statistics at Boston University. My title was research statistician. Soon after, I enrolled in a graduate program in computer science at New York University, where I completed a master’s degree. My roles in the industry have included leadership positions in statistics, data management, statistical programming, and health economics. In all my positions, I have achieved efficiencies by means of standardization and application of relevant computer technologies.
What advice would you offer younger statisticians and students who might be interested in working for the government?
OB is a very rewarding place to deploy skills in biostatistics, statistical programming, and project management. By many measures, OB offers a great opportunity to apply the most sophisticated techniques in biostatistical analysis. Our statisticians serve to protect the American public by ensuring that safe and effective drugs are available.
We review thousands of new protocols every year submitted by sponsors from all over the world. In addition, we develop new methods of analysis and write guidance documents for industry. Our staff is expected to stay current on all relevant developments, to participate in the activities of professional societies, and to give presentations at technical conferences. Newly hired staff are mentored by senior staff and collaborate broadly with other regulatory scientists.
Part of FDA’s mission is to ensure the safety and efficacy of new drugs and monitor existing products. Regulatory statisticians play a vital role in analyzing a constant stream of new data to carry out its objectives.
Working at OB has many characteristics of academic research. Statisticians are not only reviewing state-of-the-art regulatory submissions, but also developing fundamentally new statistical approaches. The breadth of analysis is that of the entire pharmaceutical industry.
The newly hired statisticians are assigned a senior mentor to introduce them to the regulatory environments, the review process, and their role in developing new methods of analysis. Many of our new statisticians participated in the ORISE (Oak Ridge Institute for Science and Education) summer internship program, where their mentoring began.
How does one find out about opportunities for internships and employment at FDA?
There are many avenues to find a job at FDA. Graduate students would be particularly interested in the ORISE summer internship program.
ORISE Research Participation Training Program
The goal of the ORISE Research Participation Training Program is to provide practical scientific training for scientists and physicians through the performance of scientific projects onsite at the FDA. All the projects are relevant to the FDA’s mission.
Office of Biostatistics Recruitment Program
The Office of Biostatistics recruits statisticians through a continual search for qualified master’s- and doctoral-degree candidates who are interested in statistical methods in clinical trial conduct and evaluation, risk assessment, pharmacovigilance (statistical reviewers), and statistical programming (statistical analysts).
Information about employment with the FDA is available on the FDA and CDER websites.
Additional information may be obtained by emailing CDER-OTS-OB-Recruitment@fda.hhs.gov.
Please tell us about your personal interests or hobbies.
I enjoy spending time with family, boating in New England, and traveling widely. Recently, we explored a number of the western national parks.