Celebrating the 40th Anniversary of the Biopharmaceutical Section: The Early Years (1966–1990)
Richard C. Zink, Lexitas Pharma Services, and Meijing Wu, AbbVie
Over its long history, the Biopharmaceutical (BIOP) Section has fostered community and created a shared sense of purpose among statisticians in the medical product industry. The year 2021 marks the 40th anniversary of BIOP. It is a time to reflect on how the section has grown from its humble roots, a time to celebrate the activities and accomplishments of the section as it seeks to support its members, and a time to envision how the section will continue to lead the way to address the challenges of medical product development in the 21st century.
In the mid-to-late 1950s, thalidomide was shown to be well-tolerated, effective as a sedative, and effective as an anti-nausea medication for pregnant women suffering from morning sickness. Despite claims that thalidomide was a nontoxic medication, with no side effects, and completely safe for pregnant women, the drug became associated with instances of peripheral neurosis and its ability to cause birth defects, according to John Frisbee in his 1990 article, “Thalidomide,” published in the Encyclopedia of Women’s Health. The thalidomide disaster in other countries led to the 1962 Kefauver-Harris amendments of the 1938 Food, Drug, and Cosmetic Act in the United States. Notable authorities granted to the US Food and Drug Administration (FDA) by this act include the following:
- The requirement that manufacturers prove effectiveness of drugs prior to marketing and disclose safety issues after marketing
- The requirement that evidence be generated by adequate and well-controlled clinical studies
- The mandate to review medications approved between 1938–1962
These new requirements necessitated the hiring of a large number of statisticians, the development of statistical departments within pharmaceutical companies, and the training of statisticians about topics in the health care field. These statisticians found themselves in need of a professional venue to develop and share methodology and promote the field, according to Robert L. Davis, S. Michael Free, Stephen W. Gulyas, Martha S. Hearron, Stacy R. Lindborg, Robert O’Neill, and Charles B. Sampson in “The History of the Biopharmaceutical Section of the American Statistical Association (ASA) 1966–1988,” published in Biopharmaceutical Report in 2005.
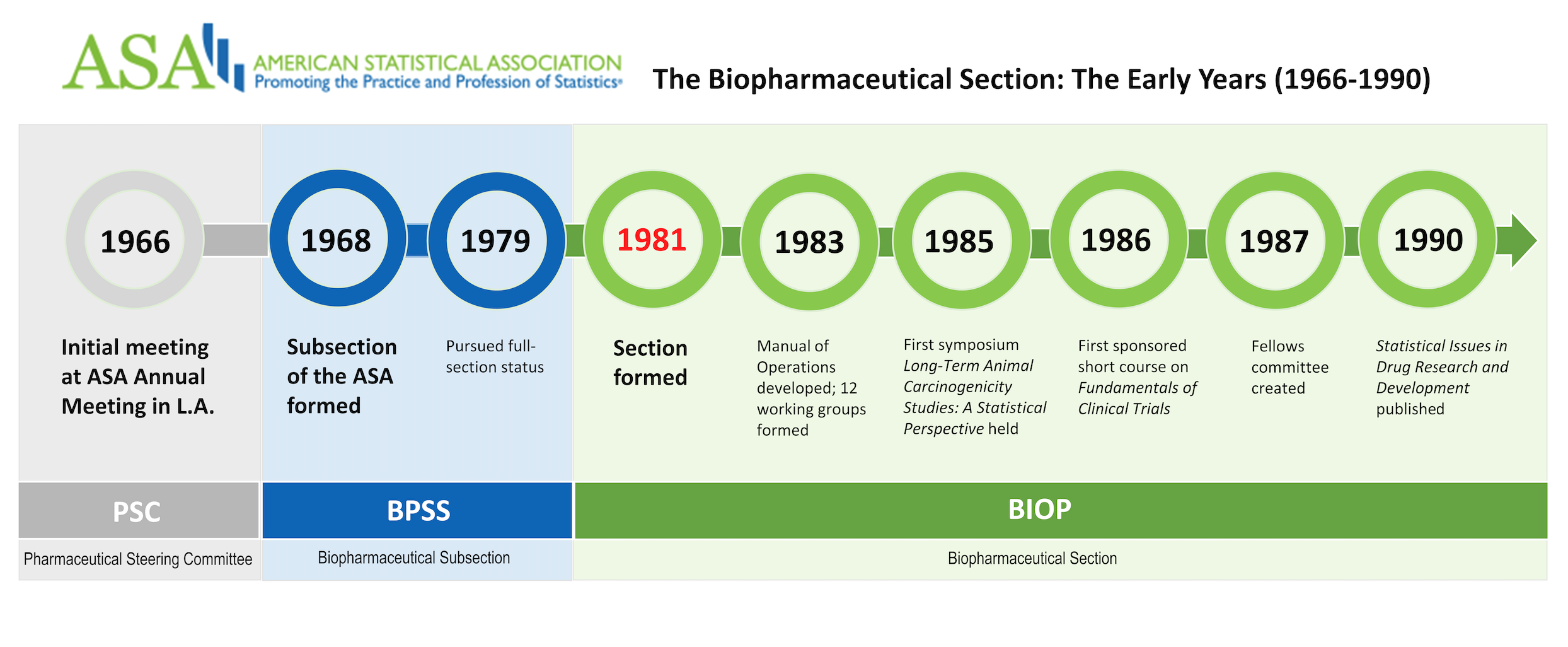
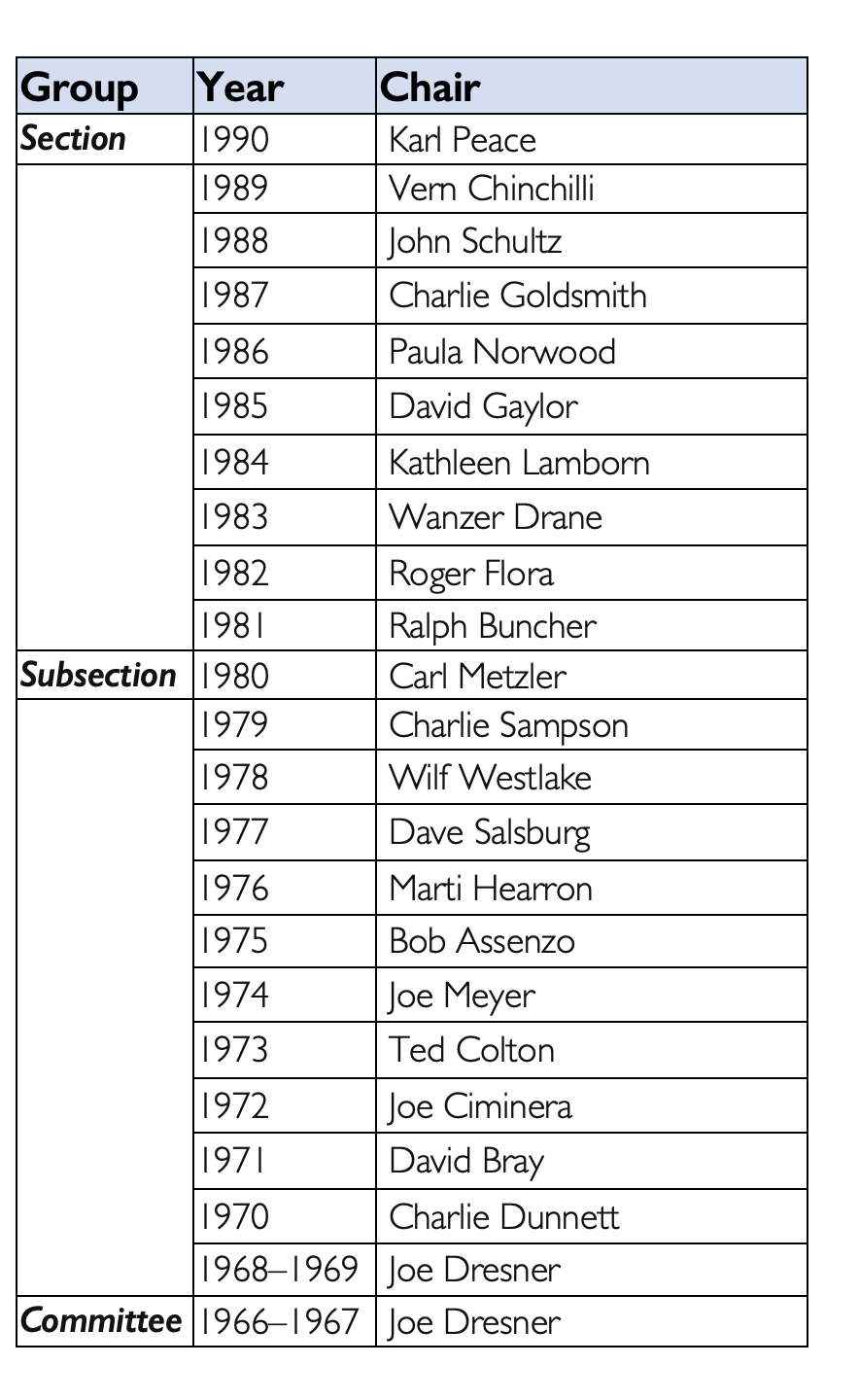
A formal group was initially discussed in 1966 at the ASA Annual Meeting in Los Angeles. Spencer M. Free, in “Some History for the Biopharmaceutical Section,” published in The American Statistician in 1990, recalled the individuals present identified five professional organizations that could serve as a home for the new group. Over time, the choices narrowed to the ASA and Drug Information Association (DIA). Eventually, the Pharmaceutical Steering Committee (PSC) petitioned to join as the Pharmaceutical Subsection of the Biometrics Section of the ASA in 1968. The name was quickly changed to the Biopharmaceutical Subsection (BPSS) to help distinguish the group from the Pharmaceutical Manufacturers Association.
BPSS was successful, and it quickly grew from 100 statisticians in 1966 to approximately 1,500 in 1979. Not surprisingly, the need for full section status was raised by the mid-1970s, and it involved many of the same culprits we see today in the birth of new sections: an active and large group, an ever-increasing scope of topics, the need for greater influence, the realization that there were potential fellows in BPSS who were not receiving proper consideration, and the increased frustration over space and visibility at conferences and meetings.
The year-long effort of petitioning the membership of BPSS, the Biometrics Section (generally opposed to the split), and the larger ASA began in 1979. Both sides presented their arguments at the February 1, 1980, board of directors meeting and a subsequent vote was in favor of the new section. A letter dated February 8 from ASA Executive Director Fred Leone to the current chair, past chair, and chair-elect of the Biometrics Section stated the question of full section status would be added to the national ballot in May of 1980.
 The new Biopharmaceutical Section would come into existence on January 1, 1980, if 500 ASA members agreed to join. The Biopharmaceutical Section became the eighth section after Biometrics (1938), Statistics and Data Science Education (1948), Business and Economic Statistics (1950), Social Statistics (1953), Physical and Engineering Sciences (1954), Statistical Computing (1972), and Survey Research Methods (1978).
The new Biopharmaceutical Section would come into existence on January 1, 1980, if 500 ASA members agreed to join. The Biopharmaceutical Section became the eighth section after Biometrics (1938), Statistics and Data Science Education (1948), Business and Economic Statistics (1950), Social Statistics (1953), Physical and Engineering Sciences (1954), Statistical Computing (1972), and Survey Research Methods (1978).
Two notable events occurred early in the first decade (1981–1990). The first edition of Statistics in the Pharmaceutical Industry, edited by C. Ralph Buncher and Jia-Yeong Tsay, was published in 1981. Second, a reorganization occurred at FDA to form the Center for Drugs and Biologics. Both events would go a long way toward clarifying the role of the professional statistician in the medical product industry and strengthening the relationships between industry and regulatory statisticians, according to Davis et al.
The first decade also saw the beginnings of many of the activities and policies still around today. For example, the Manual of Operations, which details the roles and responsibilities for section officers and committees was developed in 1983; it continues to be updated annually. Further, a series of 12 working groups were formed on various topics specific to the medical product industry. Their work would eventually be published as Statistical Issues in Drug Research and Development, edited by Karl E. Peace. The section has a formalized process for forming scientific working groups today.
BIOP held its first symposium in 1985, titled, “Long-Term Animal Carcinogenicity Studies: A Statistical Perspective.” The section would eventually go on to sponsor the annual Regulatory-Industry Statistics Workshop (RISW) and biennial Nonclinical Biostatistics Conference (NCB).
The year 1986 saw the first BIOP-sponsored short course, “Fundamentals in Clinical Trials.” BIOP-sponsored short courses are a regular feature at the Joint Statistical Meetings (JSM), RISW, and NCB today.
In 1987, a fellows committee was created to help support BIOP members interested in achieving this distinction; this committee exists today and recently published an article in Amstat News.
BIOP celebrated the ASA’s sesquicentennial (150th anniversary) in 1989 and continues to celebrate important ASA milestones today.
These earlier years were foundational in starting BIOP on a path to offering many of the services and activities members now enjoy.
There are numerous activities in the works for 2021. Panel discussions among past chairs are planned for both JSM in Seattle, Washington, and RISW in Rockville, Maryland. The mixer during the open business meeting at JSM and the mixer at RISW will feature food, fun, and reminiscing. The Biopharmaceutical Report will feature four quarterly articles highlighting the activities and successes of BIOP over 10-year increments. Finally, be on the lookout for activities and podcasts broadcast through ASA Connect throughout the year. While we are planning for in-person activities, we will adapt as needed due to any lingering safety concerns surrounding COVID-19.
To learn about more recent section history, visit the History page on the BIOP website.